-
EU approves smallpox vaccine for use against monkeypox
The Danish drugmaker that developed the jab said on Monday (July 25), the European Commission has approved a smallpox vaccine for use against monkeypox after the World Health Organization declared monkeypox a global health emergency.
Bavarian Nordic said in a statement: “The European Commission has extended the marketing authorization for the company’s smallpox vaccine, Imvanex, to include protection from monkeypox” in line with a recommendation by the EU’s medicines watchdog.
“The approval... is valid in all European Union Member States as well as in Iceland, Liechtenstein, and Norway.”
On Saturday (July 23), the WHO declared the monkeypox outbreak, which has affected nearly 16,000 people in 72 countries, to be a global health emergency — the highest alarm it can sound, the Arabnews reported, citing the AFP.
It said, Imvanex has been approved in the EU since 2013 for the prevention of smallpox.
It was also considered a potential vaccine for monkeypox because of the similarity between the monkeypox virus and the smallpox virus.
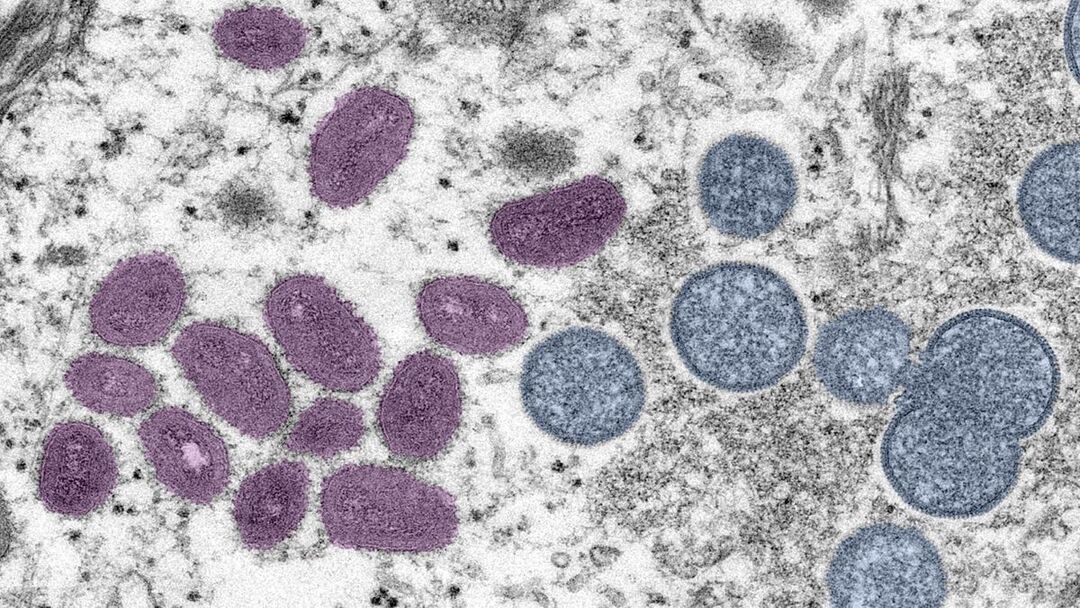
Monkeypox is less dangerous and contagious than smallpox, which was eradicated in 1980.
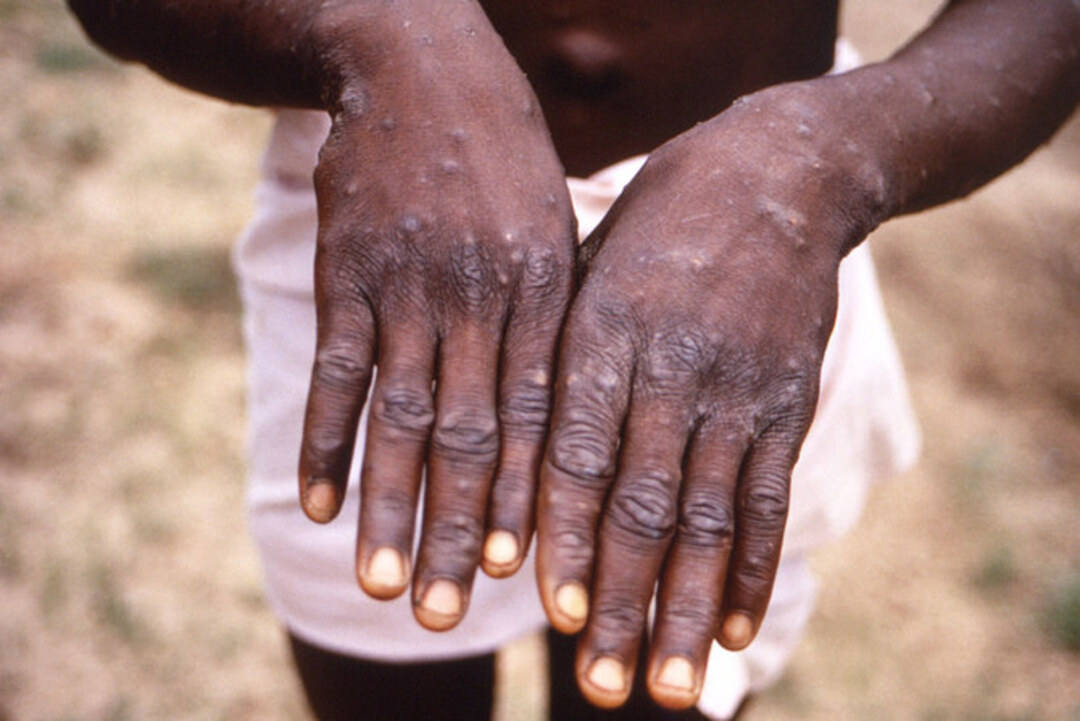
The first symptoms of monkeypox are fever, headaches, muscle pain and back pain during the course of five days.
WHO declares monkeypox a global health emergency
Rashes subsequently appear on the face, the palms of hands and soles of feet, followed by lesions, spots and finally scabs.
A surge in monkeypox infections has been reported since early May outside the West and Central African countries where the disease has long been endemic.
The EMA carries out a scientific assessment of drugs and gives a recommendation on whether any medicine should be marketed or not.
EU drug regulator recommends authorizing vaccine for monkeypox
However, under EU law the EMA has no authority to actually permit marketing in the different EU countries. It is the European Commission which is the authorizing body and takes a legally binding decision based on EMA’s recommendation.
Source: arabnews
You May Also Like
Popular Posts
Caricature
Syrians' concerns now
- December 10, 2024
Syrians' concerns now #Syria
#Bashar_al-Assad
#Liberation_of_Syria
#Syrians
#Future_of_Syria
#Levant_News

opinion
Report
ads
Newsletter
Subscribe to our mailing list to get the new updates!





















