-
Denmark reports two cases of blood clots, cerebral hemorrhage after AstraZeneca shot
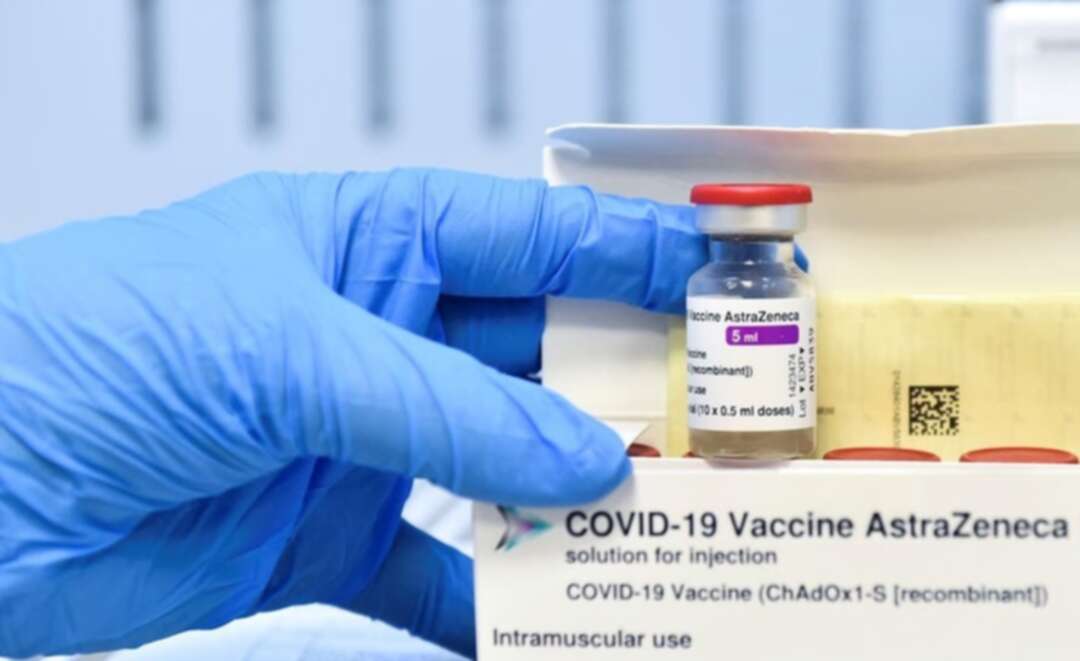
Denmark on Saturday reported two cases of hospital staff with blood clots and cerebral hemorrhage after receiving the AstraZeneca COVID-19 vaccination.
The Capital Region of Denmark, the authority that runs public hospitals in Copenhagen, said that one of the hospital staff had died and both had received the AstraZeneca vaccine less than 14 days before getting ill.
The Danish medicines agency confirmed it had received two ‘serious reports’, without giving further details.
There were no details of when the hospital staff got ill.
Some countries including Germany and France this week reversed their decision to temporarily pause use of the AstraZeneca vaccine after reports of cases of rare brain blood clots sent scientists and governments scrambling to determine any link.
Denmark, which put use of the vaccine on hold on March 11, has not yet resumed use.
The European Union’s drug watchdog said on Thursday it is still convinced the benefits of the vaccine outweigh the risks following an investigation into reports of blood clots that prompted more than a dozen nations to suspend its use.
European Medicines Agency (EMA) director Emer Cooke said on Thursday the watchdog could not definitively rule out a link between blood clot incidents and the vaccine in its investigation into 30 cases of a rare blood clotting condition.
But she said the “clear” conclusion of the review was that the benefits in protecting people from the risk of death or hospitalization outweighs the possible risks. The issue deserves further analysis, the EMA said.
AstraZeneca, which developed the shot with Oxford University, has said a review covering more than 17 million people who had received its shots in the EU and Britain had found no evidence of an increased risk of blood clots.
source: Reuters
Image source: Reuters
Levant
You May Also Like
Popular Posts
Caricature
BENEFIT AGM approves 10%...
- March 27, 2025
BENEFIT, the Kingdom’s innovator and leading company in Fintech and electronic financial transactions service, held its Annual General Meeting (AGM) at the company’s headquarters in the Seef District.
During the meeting, shareholders approved all items listed on the agenda, including the ratification of the minutes of the previous AGM held on 26 March 2024. The session reviewed and approved the Board’s Annual Report on the company’s activities and financial performance for the fiscal year ended 31 December 2024, and the shareholders expressed their satisfaction with the company’s operational and financial results during the reporting period.
The meeting also reviewed the Independent External Auditor’s Report on the company’s consolidated financial statements for the year ended 31 December 2024. Subsequently, the shareholders approved the audited financial statements for the fiscal year. Based on the Board’s recommendation, the shareholders approved the distribution of a cash dividend equivalent to 10% of the paid-up share capital.
Furthermore, the shareholders endorsed the allocation of a total amount of BD 172,500 as remuneration to the members of the Board for the year ended 31 December 2024, subject to prior clearance by related authorities.
The extension of the current composition of the Board was approved, which includes ten members and one CBB observer, for a further six-month term, expiring in September 2025, pending no objection from the CBB.
The meeting reviewed and approved the Corporate Governance Report for 2024, which affirmed the company’s full compliance with the corporate governance directives issued by the CBB and other applicable regulatory frameworks. The AGM absolved the Board Members of liability for any of their actions during the year ending on 31st December 2024, in accordance with the Commercial Companies Law.
In alignment with regulatory requirements, the session approved the reappointment of Ernst & Young (EY) as the company’s External Auditors for the fiscal year 2025, covering both the parent company and its subsidiaries—Sinnad and Bahrain FinTech Bay. The Board was authorised to determine the external auditors’ professional fees, subject to approval from the CBB, and the meeting concluded with a discussion of any additional issues as per Article (207) of the Commercial Companies Law.
Speaking on the company’s performance, Mr. Mohamed Al Bastaki, Chairman BENEFIT , stated: “In terms of the financial results for 2024, I am pleased to say that the year gone by has also been proved to be a success in delivering tangible results. Growth rate for 2024 was 19 per cent. Revenue for the year was BD 17 M (US$ 45.3 Million) and net profit was 2 Million ($ 5.3 Million).
Mr. Al Bastaki also announced that the Board had formally adopted a new three-year strategic roadmap to commence in 2025. The strategy encompasses a phased international expansion, optimisation of internal operations, enhanced revenue diversification, long-term sustainability initiatives, and the advancement of innovation and digital transformation initiatives across all service lines.
“I extend my sincere appreciation to the CBB for its continued support of BENEFIT and its pivotal role in fostering a stable and progressive regulatory environment for the Kingdom’s banking and financial sector—an environment that has significantly reinforced Bahrain’s standing as a leading financial hub in the region,” said Mr. Al Bastaki. “I would also like to thank our partner banks and valued customers for their trust, and our shareholders for their ongoing encouragement. The achievements of 2024 set a strong precedent, and I am confident they will serve as a foundation for yet another successful and impactful year ahead.”
Chief Executive of BENEFIT; Mr. Abdulwahed AlJanahi commented, “The year 2024 represented another pivotal chapter in BENEFIT ’s evolution. We achieved substantial progress in advancing our digital strategy across multiple sectors, while reinforcing our long-term commitment to the development of Bahrain’s financial services and payments landscape. Throughout the year, we remained firmly aligned with our objective of delivering measurable value to our shareholders, strategic partners, and customers. At the same time, we continued to play an active role in enabling Bahrain’s digital economy by introducing innovative solutions and service enhancements that directly address market needs and future opportunities.”
Mr. AlJanahi affirmed that BENEFIT has successfully developed a robust and well-integrated payment network that connects individuals and businesses across Bahrain, accelerating the adoption of emerging technologies in the banking and financial services sector and reinforcing Bahrain’s position as a growing fintech hub, and added, “Our achievements of the past year reflect a long-term vision to establish a resilient electronic payment infrastructure that supports the Kingdom’s digital economy. Key developments in 2024 included the implementation of central authentication for open banking via BENEFIT Pay”
Mr. AlJanahi concluded by thanking the Board for its strategic direction, the company’s staff for their continued dedication, and the Central Bank of Bahrain, member banks, and shareholders for their valuable partnership and confidence in the company’s long-term vision.
opinion
Report
ads
Newsletter
Subscribe to our mailing list to get the new updates!





















