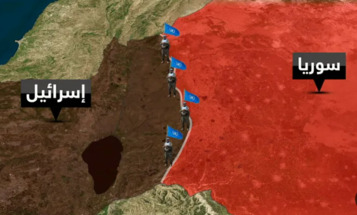
-
UK pledges £29m more to fast-track vaccines against Covid variants

Money for Porton Down laboratory will help to ‘future-proof’ country, says Matt Hancock
The UK government is pledging extra money to fast-track vaccines in an effort to stay “one step ahead” of coronavirus variants.
The multimillion-pound investment in testing facilities at Porton Down in Wiltshire would help to “future-proof” the country, said the health secretary, Matt Hancock.
The government is pledging an additional £29.3m, on top of £19.7m already promised.
Scientists at the Porton Down research laboratory test blood samples to monitor the effectiveness of coronavirus vaccines. Current testing capacity is 700 tests a week, but it is increasing to 1,500 by January 2022.
The extra funding would double the capacity for testing variant samples to 3,000 a week when work at the site was completed, said the Department of Health and Social Care.
“We’ve backed UK science from the very start of this pandemic and this multimillion-pound funding for a state-of-the-art vaccine testing facility at Porton Down will enable us to further future-proof the country from the threat of new variants,” said Hancock.
Dr Jenny Harries, chief executive at the UK Health Security Agency, said a new variant that could resist vaccines was the “greatest risk” of a third wave of the virus sweeping the country. “This new investment will help us stay one step ahead of the virus by doubling our capacity to test vaccine effectiveness against emerging variants,” she said.
The government is making plans for a booster Covid vaccine programme in the autumn, to protect the most vulnerable before winter, and last week ordered a further 60m doses of the Pfizer/BioNTech vaccine.
The vaccines minister, Nadhim Zahawi, said the UK was carrying out about 50% of the world’s genome sequencing of the virus and its mutations, and had done a deal with Germany’s CureVac to develop the next generation of vaccines targeting new emerging variants in the pandemic, which would include keeping a “library” of the virus.
“We need to make sure that we have vaccine variants that are ready for any virus variant that may escape,” Zahawi told Sky News. “The good news is the current vaccination programme and the vaccines we’re deploying are working effectively against the dominant virus in the United Kingdom.”
He added: “It’s all about making sure we back our scientists, we back Public Health England, we back the team of Porton Down who have done an amazing job throughout this pandemic.
“The principle of this is to future-proof the vaccination programme for the autumn, and then for years to come. Because as we move, hopefully from pandemic to endemic, this will be similar to the annual flu vaccination programme where the scientists will decide as they do it <…> which variants are the most concern and then we eventually formulate of a vaccine to deal with those.”
Zahawi said by the autumn over-50s could have booster shots against the coronavirus, telling BBC Radio 4’s Today programme: “You could have a shot in one arm for flu and the other for coronavirus.”
The Times reported on Wednesday that a third jab was to be offered to everyone over 50 in autumn, with trials of two options, supervised by Chris Whitty, the chief medical officer for England, under way.
The first involves vaccines specifically modified to tackle new variants, while the second would involve a third shot of one of the three versions already in use: Pfizer/BioNTech, Oxford/AstraZeneca or Moderna.
Zahawi said the government was to give experts “as many options as possible”, adding that vaccine manufacturing capacity would increase to about 600m doses a year by next year. This would include vaccines made in Livingston in West Lothian, Oxfordshire, Braintree and Stockton-on-Tees with the final “fill and finish” step at GlaxoSmithKline’s plant in Barnard Castle, County Durham.
source: Alexandra Topping
Levant
You May Also Like
Popular Posts
Caricature
BENEFIT Sponsors BuildHer...
- April 23, 2025
BENEFIT, the Kingdom’s innovator and leading company in Fintech and electronic financial transactions service, has sponsored the BuildHer CityHack 2025 Hackathon, a two-day event spearheaded by the College of Engineering and Technology at the Royal University for Women (RUW).
Aimed at secondary school students, the event brought together a distinguished group of academic professionals and technology experts to mentor and inspire young participants.
More than 100 high school students from across the Kingdom of Bahrain took part in the hackathon, which featured an intensive programme of training workshops and hands-on sessions. These activities were tailored to enhance participants’ critical thinking, collaborative problem-solving, and team-building capabilities, while also encouraging the development of practical and sustainable solutions to contemporary challenges using modern technological tools.
BENEFIT’s Chief Executive Mr. Abdulwahed AlJanahi, commented: “Our support for this educational hackathon reflects our long-term strategic vision to nurture the talents of emerging national youth and empower the next generation of accomplished female leaders in technology. By fostering creativity and innovation, we aim to contribute meaningfully to Bahrain’s comprehensive development goals and align with the aspirations outlined in the Kingdom’s Vision 2030—an ambition in which BENEFIT plays a central role.”
Professor Riyadh Yousif Hamzah, President of the Royal University for Women, commented: “This initiative reflects our commitment to advancing women in STEM fields. We're cultivating a generation of creative, solution-driven female leaders who will drive national development. Our partnership with BENEFIT exemplifies the powerful synergy between academia and private sector in supporting educational innovation.”
Hanan Abdulla Hasan, Senior Manager, PR & Communication at BENEFIT, said: “We are honoured to collaborate with RUW in supporting this remarkable technology-focused event. It highlights our commitment to social responsibility, and our ongoing efforts to enhance the digital and innovation capabilities of young Bahraini women and foster their ability to harness technological tools in the service of a smarter, more sustainable future.”
For his part, Dr. Humam ElAgha, Acting Dean of the College of Engineering and Technology at the University, said: “BuildHer CityHack 2025 embodies our hands-on approach to education. By tackling real-world problems through creative thinking and sustainable solutions, we're preparing women to thrive in the knowledge economy – a cornerstone of the University's vision.”
opinion
Report
ads
Newsletter
Subscribe to our mailing list to get the new updates!