-
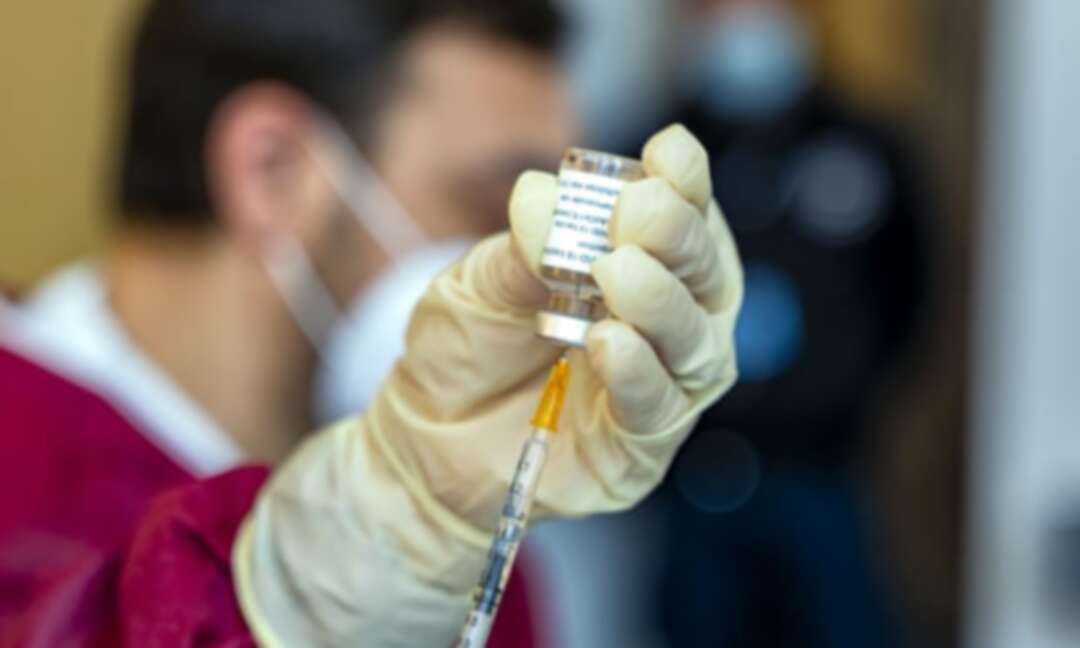
UK study on mixing Covid vaccines between jabs to be expanded
Researchers to examine whether mixing vaccines may give longer-lasting immunity against virus
A major UK study examining whether Covid vaccines can be safely mixed with different types of jabs for the first and second doses is to be expanded.
Researchers running the Com-Cov study, launched in February to investigate alternating doses of the Oxford/AstraZeneca and Pfizer vaccines for the first and second doses, will now include a shot of the Moderna or Novavax vaccines.
The study is examining whether mixing vaccines may give broader, longer-lasting immunity against the virus and new variants, and offer more flexibility in the administration of vaccines.
Led by the University of Oxford, the study will seek to recruit adults aged over 50 who have received their first vaccination in the past eight to 12 weeks.
Matthew Snape, an associate professor in paediatrics and vaccinology at the University of Oxford, who is chief investigator on the trial, said: “The focus of both this and the original Com-Cov study is to explore whether the multiple Covid-19 vaccines that are available can be used more flexibly, with different vaccines being used for the first and second dose.
“If we can show that these mixed schedules generate an immune response that is as good as the standard schedules, and without a significant increase in the vaccine reactions, this will potentially allow more people to complete their Covid-19 immunisation course more rapidly.
“This would also create resilience within the system in the event of a shortfall in the availability of any of the vaccines in use.”
The volunteers, who will have received either the Oxford/AstraZeneca or Pfizer/BioNTech vaccine, will be randomly allocated to receive either the same vaccine for their second dose or a dose of the jabs produced by Moderna or Novavax.
The Moderna jab has started being rolled out across the UK, and the Novavax jab manufactured by GlaxoSmithKline (GSK) is under review by the Medicines and Healthcare products Regulatory Agency (MHRA).
The six new arms of the trial will each recruit 175 candidates, adding 1,050 recruits to the programme. The researchers will study any adverse reactions and the immune system responses to these new combinations of vaccines.
If the study shows promising results, the MHRA and Joint Committee on Vaccination and Immunisation (JCVI) would formally assess the safety and efficacy of any new vaccination regimen before it is rolled out to patients.
Snape said he hoped the results of the second part of the study would be available in June or July, with the first part expected to report results next month.
He told a press briefing: “What I’m hoping is that we won’t rule out any combinations. That’s how we need to look at it – are there any combinations we shouldn’t be giving because they don’t generate a good immune response, and I’m hoping that won’t be the case.
“And that will give us lots of flexibility, not just in the UK, not just in Europe where we’re looking about restricting uses of some vaccines for some age groups, but across the world, where we have, perhaps, a little bit more intermittent supply of vaccines, not as reliable.”
Prof Jeremy Brown, a member of the JCVI, said people would eventually have to mix Covid-19 jabs.
He told BBC Radio 4’s Today programme: “It’s practically going to have to be that way because, once you’ve completed a course of, say, the Moderna or Pfizer or the AstraZeneca with two doses, in the future it’s going to be quite difficult to guarantee you get the same type of vaccine again.”
source: Sarah Marsh
Levant
You May Also Like
Popular Posts
Caricature
BENEFIT Sponsors BuildHer...
- April 23, 2025
BENEFIT, the Kingdom’s innovator and leading company in Fintech and electronic financial transactions service, has sponsored the BuildHer CityHack 2025 Hackathon, a two-day event spearheaded by the College of Engineering and Technology at the Royal University for Women (RUW).
Aimed at secondary school students, the event brought together a distinguished group of academic professionals and technology experts to mentor and inspire young participants.
More than 100 high school students from across the Kingdom of Bahrain took part in the hackathon, which featured an intensive programme of training workshops and hands-on sessions. These activities were tailored to enhance participants’ critical thinking, collaborative problem-solving, and team-building capabilities, while also encouraging the development of practical and sustainable solutions to contemporary challenges using modern technological tools.
BENEFIT’s Chief Executive Mr. Abdulwahed AlJanahi, commented: “Our support for this educational hackathon reflects our long-term strategic vision to nurture the talents of emerging national youth and empower the next generation of accomplished female leaders in technology. By fostering creativity and innovation, we aim to contribute meaningfully to Bahrain’s comprehensive development goals and align with the aspirations outlined in the Kingdom’s Vision 2030—an ambition in which BENEFIT plays a central role.”
Professor Riyadh Yousif Hamzah, President of the Royal University for Women, commented: “This initiative reflects our commitment to advancing women in STEM fields. We're cultivating a generation of creative, solution-driven female leaders who will drive national development. Our partnership with BENEFIT exemplifies the powerful synergy between academia and private sector in supporting educational innovation.”
Hanan Abdulla Hasan, Senior Manager, PR & Communication at BENEFIT, said: “We are honoured to collaborate with RUW in supporting this remarkable technology-focused event. It highlights our commitment to social responsibility, and our ongoing efforts to enhance the digital and innovation capabilities of young Bahraini women and foster their ability to harness technological tools in the service of a smarter, more sustainable future.”
For his part, Dr. Humam ElAgha, Acting Dean of the College of Engineering and Technology at the University, said: “BuildHer CityHack 2025 embodies our hands-on approach to education. By tackling real-world problems through creative thinking and sustainable solutions, we're preparing women to thrive in the knowledge economy – a cornerstone of the University's vision.”
opinion
Report
ads
Newsletter
Subscribe to our mailing list to get the new updates!