-
US recommends ‘pause’ for Johnson & Johnson COVID-19 vaccine over clot reports
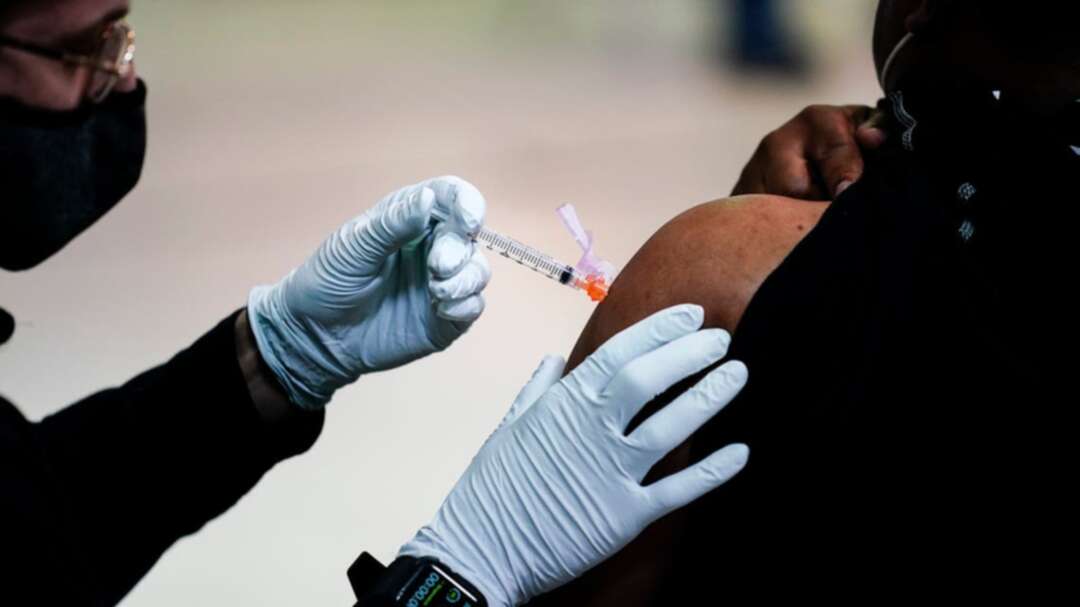
The United States is recommending a “pause” in administration of the single-dose Johnson & Johnson COVID-19 vaccine to investigate reports of potentially dangerous blood clots.
In a joint statement Tuesday, the Centers for Disease Control and Prevention and the Food and Drug Administration said it was investigating clots in six women in the days after vaccination, in combination with reduced platelet counts. More than 6.8 million doses of the J&J vaccine have been administered in the US.
US federal distribution channels, including mass vaccination sites, will pause the use of the J&J shot, and states and other providers are expected to follow.
CDC’s Advisory Committee on Immunization Practices will meet Wednesday to discuss the cases and the FDA has also launched an investigation of the cases.
“Until that process is complete, we are recommending a pause in the use of this vaccine out of an abundance of caution,” Dr. Anne Schuchat, Principal Deputy Director of the CDC and Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research said in a joint statement.
source: The Associated Press
Image source: AP
Levant
You May Also Like
Popular Posts
Caricature
BENEFIT Sponsors Gulf Uni...
- April 17, 2025
BENEFIT, the Kingdom’s innovator and leading company in Fintech and electronic financial transactions service, has announced its sponsorship of the “Innovation and Sustainable Technology Solutions Competition (GU - IST Solutions), hosted by Gulf University at its main campus.
This strategic sponsorship reflects BENEFIT’s active role in advancing technological innovation and fostering sustainable solutions to future challenges. It also seeks to empower Bahraini youth by enhancing their skills, capabilities, and competitiveness in innovation and solution development—contributing meaningfully to the broader goals of sustainable development across all sectors.
As part of BENEFIT’s active involvement in the competition, the company has announced that Hanan Abdulla Hasan, Senior Manager of Public Relations and Communication, will serve on the competition’s supervisory committee. Her upcoming participation reflects BENEFIT’s forward-looking commitment to championing academic and professional excellence.
Commenting on the occasion, Hanan Abdulla Hasan, Senior Manager of Public Relations and Communication at BENEFIT, said, “We are privileged to support this pioneering initiative, which aligns seamlessly with BENEFIT’s enduring commitment to fostering innovation and nurturing the potential of Bahrain’s youth. Our participation is rooted in a deep sense of social responsibility and a firm belief in the pivotal role of innovation in shaping a sustainable future. Through such platforms, we seek to empower the next generation with the knowledge, skills, and foresight required to develop impactful solutions that address future challenges, in line with the United Nations Sustainable Development Goals 2030.”
Dr. Aseel Al Ayash Dean of the College of Engineering in Gulf University commented, “We extend our sincere gratitude to BENEFIT for their generous sponsorship and support of the Innovation and Sustainable Technology Solutions Competition. This contribution plays an instrumental role in helping us achieve the strategic goals of this initiative, namely, cultivating a culture of innovation and sustainability, encouraging efforts that address the imperatives of sustainable development, and enhancing the practical and professional capabilities of our students and participants.”
The event will bring together a diverse spectrum of participants, including secondary school students, university undergraduates, engineers, industry professionals, entrepreneurs, academic researchers, and subject matter experts representing a wide range of disciplines.
The competition seeks to inspire participants to develop and present innovative, sustainable technologies aimed at addressing pressing environmental, social, and economic challenges. It encourages the formulation of business models that integrate advanced technological solutions with core principles of sustainability. Moreover, it serves as a platform for emerging leaders, entrepreneurs, and innovators to contribute to the advancement of the Sustainable Development Goals, promote the ethos of responsible technology, and demonstrate its transformative potential across various sectors.
Attendees will have the opportunity to view a series of project presentations submitted by participants, covering diverse areas such as eco-friendly product design, smart and sustainable innovations, renewable energy technologies, water conservation and management, waste minimisation and recycling, green architectural solutions, and sustainable transportation systems. Outstanding projects will be formally recognised and awarded at the conclusion of the event.
opinion
Report
ads
Newsletter
Subscribe to our mailing list to get the new updates!





















